The Pressures and Pain Points of Hardware-Based Medical Device Development
Unlike the development of Software as a Medical Device (SaMD), medical devices that are hardware-based require a different approach to production.
Developing a medical solution with hardware components is more costly and time-consuming than developing SaMD simply because your team must translate your designs into a physical product. We are going to help you understand the expectations of hardware-based medical device production by exploring development processes and regulations.
Design Iterations for Medical Devices
Hardware development is a long process due to the number of iterations it takes to finalize and shape a product. Since engineers create physical prototypes for every iteration, development cycles are prolonged since they can take weeks or months. With more complex and distinct designs, medical device prototypes become challenging for teams meeting timely objectives.
It is important to distinguish the price difference between physical iterations and manufactured end products. Medical Device prototypes that come from design iterations are much more costly for companies since they can cause setbacks in budgets and timelines for market approval. To avoid exceeding budgets or setting back development timelines, your team should implement a Quality Management System (QMS) that ensures medical device designs are meeting specifications and effectiveness.
Verification Testing for Medical Devices
Any company developing a medical device for FDA approval must perform verification testing on their medical device prototypes in order to ensure safety and effectiveness. There is a blueprint that hardware engineers use to develop a hardware-based medical device. This blueprint is known as the Design Control, guiding hardware engineers in developing a device tied to its product specifications and requirements. The FDA requires that the design process follow this blueprint outlined in 21 CFR Part 820.30. Yet, many development teams aren’t too familiar with the regulations, let alone the requirements.
What does verification testing for hardware look like? Engineering teams must individually test and verify each prototype making sure it matches each design requirement. Having protocols for verification in place will make this process more efficient. Protocols are well-documented instructions for teams looking to verify their prototypes with thorough details.
Oftentimes, teams using a paper-based system of documentation will scramble to find the correct protocols for this phase of development. A life science QMS with document automation tools will streamline verification processes for engineering teams by clearly outlining the next steps for a device on the development timeline if they fail or pass the tests.
Manufacturing for Medical Devices
For Hardware-based medical device developers, manufacturing is a costly and time-consuming phase of development. It contains various steps that can be stressful for teams under a strict timeline.
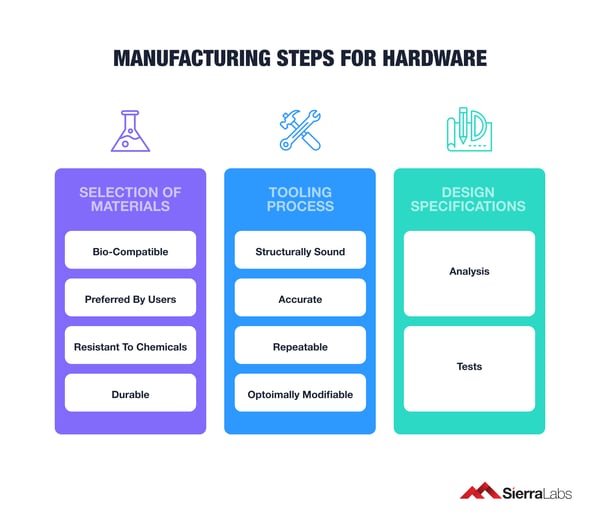
Take into consideration:
- Selection of materials are:
- Bio-compatible
- Preferred by Users
- Resistant to Chemicals
- Durable
- Tooling process must ensure parts are:
- Structurally Sound
- Accurate
- Repeatable
- Optimally Modifiable
- Design specifications are being met through:
- Analysis
- Tests
Each phase presents a new challenge to your team, particularly against efficient processes. Despite this, many companies compromise quality by speeding through each manufacturing phase in order to meet deadlines.
There is a solution to having efficiency without losing quality. Having a QMS for medical devices will help your team through every manufacturing phase by managing quality processes and confirming their satisfaction with regulatory and statutory standards related to hardware-based medical devices.
Absence of Updates for Hardware-based
In comparison to SaMDs that have updates, hardware-based medical devices can’t simply update. Developers must extract a product from the market and correct it with minimal repercussions, usually following these two options:
- Make incremental changes to a previous design.
- Issue a recall.
Both of these options are expensive and can result in reputable damages to your company.
Improve Development Processes With a QMS
Don’t worry, there is a third option. Choosing a robust medical device QMS that will not only streamline your documentation but also ensure your development processes are adhering to FDA regulations.
The optimized solution to reduce risk, maintain quality, and speed innovation is by utilizing an FDA compliant medical device quality management system application. Sierra Quality Management System (QMS) is a QMS for life sciences that allows you to easily integrate risk management into your production process.
Sierra QMS is designed for organizations that are looking to market medical devices in a global regulated environment. It is built for engineering teams to operate with their preferred tool-sets while automating compliance with medical device QMS principles for global markets.
Want to see how implementing Sierra QMS can accelerate your device's journey to market?
Click Here for a Free Demo!
It's that simple.

