Understanding the difference and benefits between ISO and IEC Standards for Software as a Medical Device

Achieving Compliance with International Frameworks
On medical device companies, road to compliance there are several international standards that they must take note of. There are currently two international organizations that decide and maintain standards and best practices for SaMD development, International Organization for Standardization (ISO) and International Electrotechnical Commission (IEC).
Due to the increased complexity of software within medical devices, a more stringent regulatory review by regulatory bodies like the FDA has prolonged time-to-markets. Luckily, this same complexity has paved a path for organizations like the FDA to adopt ISO and IEC standards that allowing medical device companies achieve conformance and establish proper protection protocols for the safety of end-users.
Further Understanding of ISO and IEC Standards for SaMD
Many medical device manufacturers expose themselves to weaknesses and vulnerabilities by not aligning their processes to ISO and IEC standards.This results in a backlog for development and more importantly, a longer time-to-market. The first general standards that your software development teams should be familiar with when developing SaMD are explained in the following image.
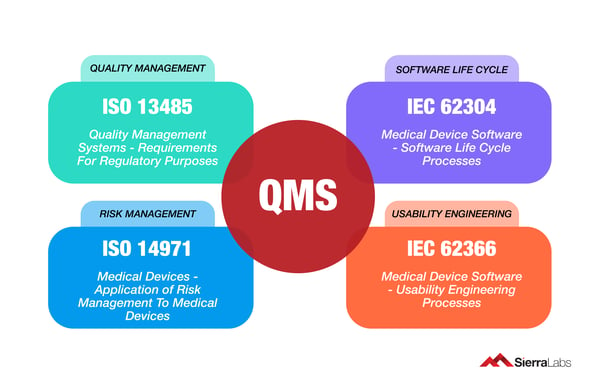
Before any SaMD team can begin developing their medical device solution, they must have a strong understanding of what and who in their team should know both these ISO standards:
| ISO Standard | Description | Who Should Know? |
| ISO 13485:2016 | Defines what requirements medical device companies must adhere to with their QMS. It is designed to respond to the latest QMS practices, including changes in technology and regulatory requirements and expectations. | Quality Team |
| ISO 14971:2019 | Defines a standard process for identifying risks associated with medical devices at all stages in a device’s life cycle, from product design to procurement to production and post-market use. | Quality Team |
When it comes to meeting IEC standards, it is important to have a quality manager that can distinguish key regulatory terminology.
| IEC Standard | Description | Who Should Know? |
| IEC 62304 | Specifies life cycle requirements for the development of medical software and can be used as a key benchmark for compliance within both US and European markets. | Software Project Manager(s) & Development Team |
| IEC 62366 | Describes the various activities such as validation testing for interface design that should be implemented in a usability engineering process during SaMD development. | Software Project Manager(s) & Development Team |
| IEC 60601-1:2 | Ensures safety and effectiveness of device network, software interfaces and electrical equipment (hardware) through a series of technical standards. | Software Project Manager(s) & Development Team |
Equip Your Team with a QMS
Now that you have a clearer understanding of ISO and IEC standards, let’s talk about ways to actually meet conformance for these standards within your organization!
Having a QMS for SaMD startups is crucial for teams to stay on track and record the development of their device from start to finish streamlining demonstration of procedures and standards like ISO and IEC. Additionally, an SaMD QMS makes FDA audits a breeze by easily demonstrating full lifecycle traceability of your products.
Sierra QMS is designed for organizations who are looking to market SaMDs in a global regulated environment. Sierra Quality Management System (QMS) allows you to easily integrate risk management into your production process and operate with your own preferred tool-sets.
Want to see how implementing Sierra QMS will help you achieve ISO and IEC Certifications?
Click Here for a Free Demo!

