Uncovering how Agile practices fulfill Software as a Medical Device (SaMD) Development
The Association for the Advancement of Medical Instrumentation (AAMI) TIR45 guidance document outlines how to implement Agile practices into your software development process while also detailing the activities of IEC 62304.
Check out our blog that breaks down AAMI TIR45 for SaMD.
Agile approaches have allowed development teams to coordinate more checkpoints improving the software’s development and its function(s). The methodology enables teams to do this while keeping patients, physicians, nurses, and other important end-users in mind.
What is IEC 62304?
IEC 62304 is a standard accepted by most global regulatory bodies, including the FDA, and deals with the medical device software lifecycle processes. Managing the lifecycle development process of software is not easy. Because of this, the standard is crucial for software developers in the medical device space.
How is Agile aiding software teams?
The high level of flexibility, creativity, and receptiveness of Agile is the reason why it is the preferred method of development for software teams in recent years. This method also aids development teams by keeping their projects organized and efficient.
Learn more about the core elements of Agile in Embrace Agile in SaMD to stay competitive!
In the Agile Manifesto, one of the main values is “working software over comprehensive documentation”. This may easily be misconstrued by software development teams as an opportunity to cut documentation standards. By doing this, your team is failing to comply with IEC 62304 standards which can affect patient safety and security in the longterm.
Regulatory Implications of IEC 62304
The regulatory implications of building software may impact more than software functionality but also patient safety, efficacy, and even privacy and security. Your team’s iterative tests and review should establish and maintain these and various additional requirements when attempting to build your software for the healthcare space.
IEC 62304 is clear and concise, your team must document and verify all activities to achieve traceability. The most important parts of your reviews should answer the following:
- Are all software requirements met? Check with traceability matrix or tools
- Is the risk management file updated? Are all risk-minimizing measures in the architecture implemented? Are risks that arise from the choice of software architecture, analyzed and controlled?
- Does the software architecture document the requirements of the development plan?
- Is the software architecture so evident that the developers can implement them without further inquiry?
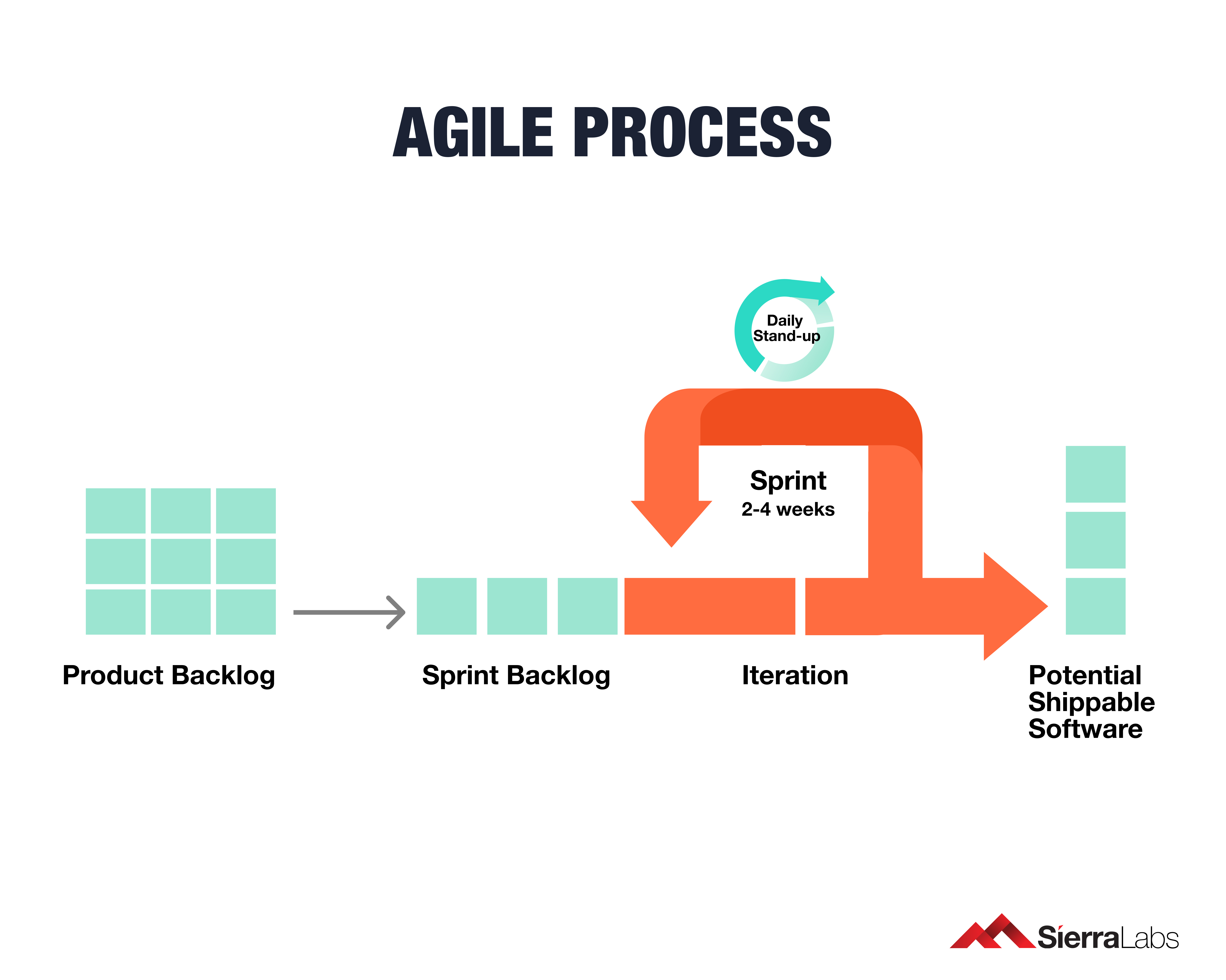
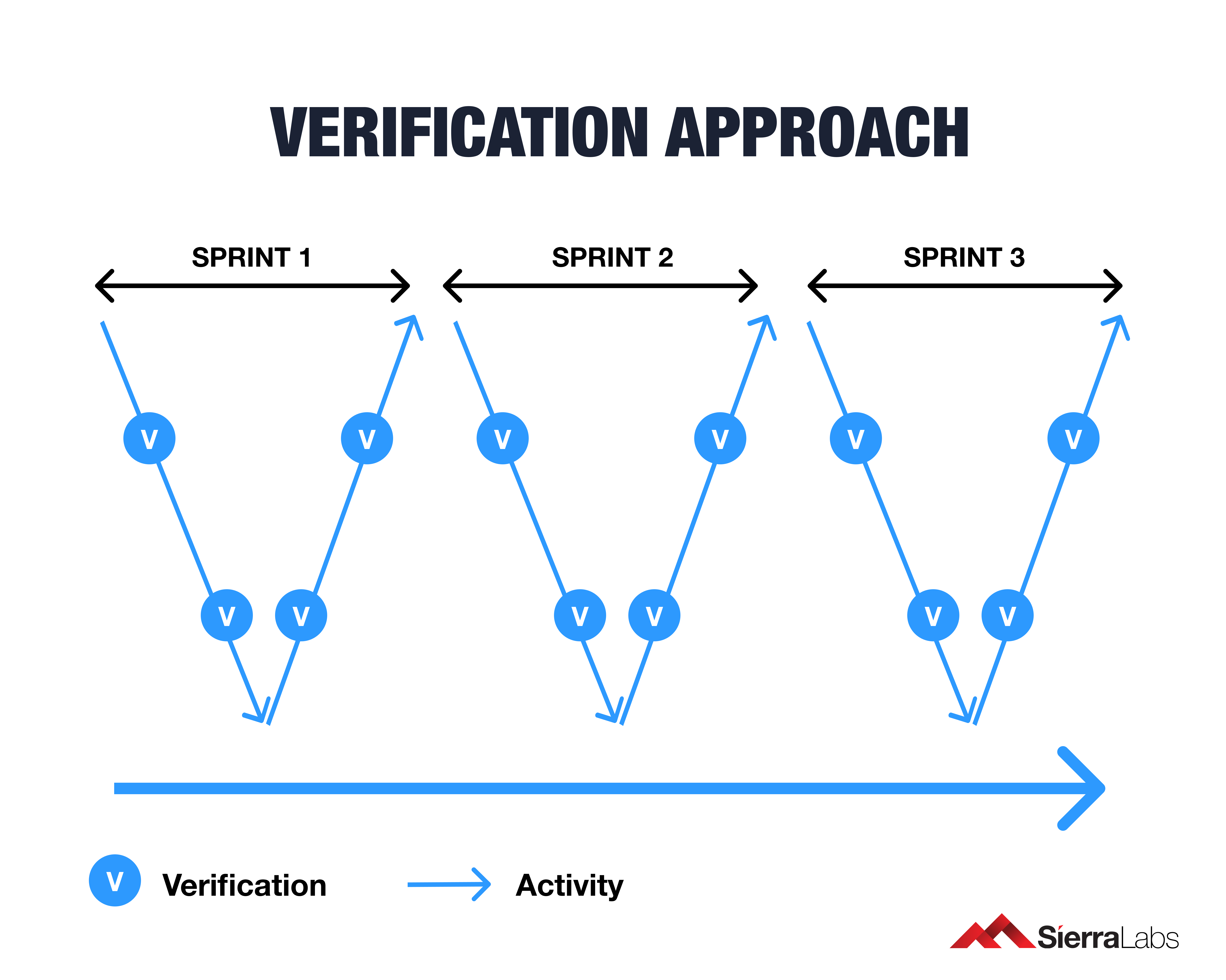
The Best Approach for Verification in Each Sprint
Agile is actually consistent with IEC 62304 as specifying requirements is reinforced through individual methods known as Sprints, which we will explore a little further.
To be agile in medical device software development, your team must reinforce the requirements of 62304 each time a Sprint is performed. The requirements include the following:
- Management and Traceability
- Design Verification
- Validation
- Code Reviews
- Integration Testing
- Risk Management and Control
- Configuration Management
With this review model, activities within a Sprint won’t backtrack your progress or your verification efforts. Verifying each Sprint aligns with the regulatory standard of document verification under IEC 62304. Any changes made such as changes in architecture or code is reviewed before the next activity can begin. This method will maximize the efficiency of approvals throughout the entire development process.
Streamline your documentation processes with QMS
Implementing an Agile Quality Management System around your existing best practices will be an advantage for your team. In addition to complying with IEC 62304 specifications, a QMS offers SaMD development teams streamlined demonstration of procedures and standards when it comes to FDA audits.
The optimized solution to reduce risk, maintain quality, and accelerate innovation is by utilizing an FDA compliant and best practices conformant medical device quality management application. Sierra Quality Management System (QMS) is a QMS for life sciences that automates regulatory compliance for teams that are looking to market their SaMD in a global regulated environment.
Sierra Labs helps both medical device software developers and SaMDs developers to build a robust QMS that includes a variety of effective workflow management tools.
Want to see how implementing Sierra QMS can accelerate
your journey to market?
Click here for a Free Trial!

