Easy Guidance for SaMD Development and Agile Practices

It is difficult to talk about the medical device space without mentioning the immense role software has played in advancing the medical field. Since the introduction of Software as a Medical Device (SaMD), regulatory standards have had to keep up with the types of development processes these devices require.
A technical information report (TIR) from the Association for the Advancement of Medical Instrumentation (AAMI), titled AAMI TIR45, offers the proper recommendations if you are developing a SaMD. This report is well-aligned with FDA guidance documents and even international standards like the EMA. Let's look into AAMI TIR45 more closely.
Defining AAMI TIR45
AAMI TIR45 is the Advancement of Medical Instrumentation's guidance on the use of Agile practices in the development of medical device software.
AAMI TIR45:2012 focuses on refuting the following objections to Agile methodology in the Medical Device space:
- Since medical devices need to be safe, effective, and reliable, the agile methodology does not have sufficient rigor and sophistication to be used in these critical systems.
- Agile methodology doesn't adhere to a central plan with controlled processes and proper documentation, required by the FDA’s Quality System Regulation (QSR).
- Even if Agile had appropriate processes to meet FDA regulations, it wouldn’t matter, because the FDA prescribes waterfall processes.
This guidance dives deep into how Agile can be used in the FDA-regulated space.
SaMD Development Methodology
It is a common misconception that FDA regulations prefer the "Waterfall" methodology for SaMD development. The FDA does not explicitly prescribe a particular development methodology via 21 CFR Part 820 Quality System Regulation. Much of the misconception derives from IEC 62304's phrasing, which recognizes "Waterfall" as the preferred method.
The Software development strategy for medical devices has traditionally operated under the “Waterfall model” which you can learn more about in our blog, Embrace Agile in SaMD to stay competitive!
Since SaMD development cycles tend to be much shorter than other medical device cycles (three to five years) there is a stronger focus on unprecedented changes. Because of this, the "Agile" model has emerged as a frontrunner for SaMD development cycles.
Defining the Changes in SaMD Development
The reality of developing complex systems with human interaction and interaction with other systems is that change is inevitable. Change can come from many sources:
- External Change
- Driven by technology, regulations, competition, or demographic shifts
- Internal Change
- Driven by the growth of a company or new product lifecycles
- Requirements Change
- Driven by emerging design or development activities to comply with structural requirements
- Design Change
- Driven by the role of user experience or testing feedback
- Development Change
- Driven by the originality of the design and implementation of the system
Agile Addresses Setbacks
"Waterfall” methods aren't meant to keep up with the pace of change and uncertainty that product development organizations are face.
Agile assumes that the requirements and design of your software will continuously evolve as product feedback comes in. The way this method works is by breaking individual projects down into user stories, segments of value derived from user functionality. Breaking down these segments into one to three-week cycles (known as iterations) will be introduce an adaptive agenda to your development process.
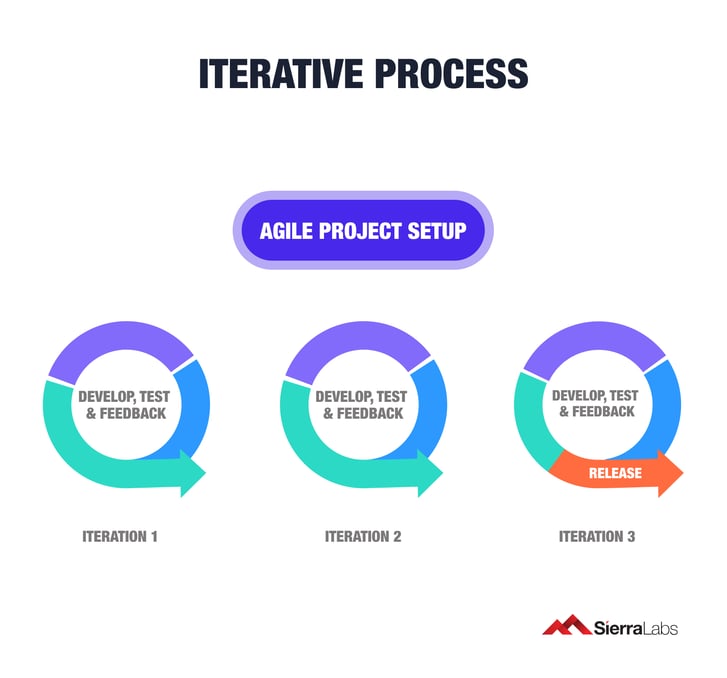
Some examples of the iterative process include: software requirements, design, testing, risk management, and customer feedback.
The Benefits of AAMI TIR45
The cost of change curve shows the relative cost of addressing a changed requirement, either because it was missed or misunderstood, at different stages of the product life cycle.
As the development process unfolds, the margin of fixing an error becomes greater and more costly to your entire organization. This is due to the characterization of a SaMD development process setup, each stage constructed on top of each one that precedes it.
The "Agile" method aims to introduce adaptive planning to a fast development process encouraging rapid and flexible responses to changes.
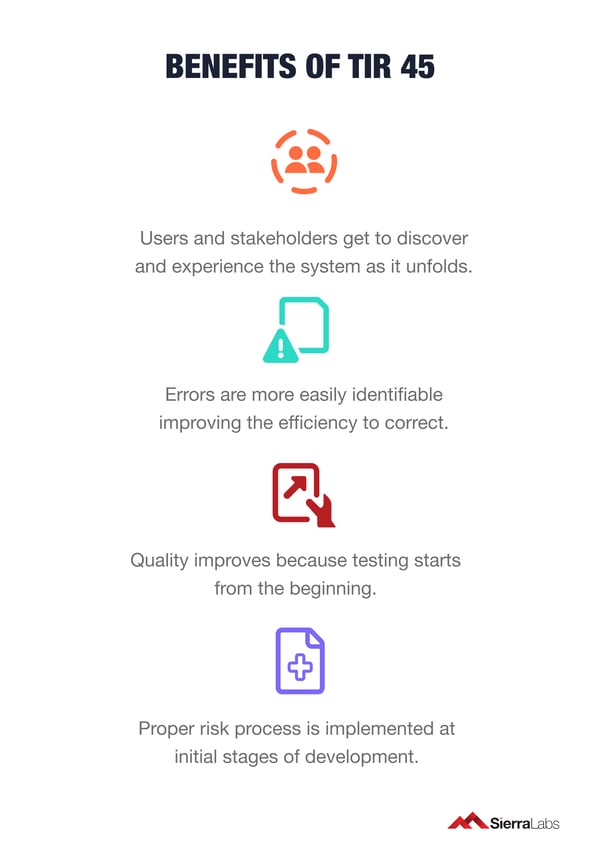
The following is a list of benefits your development team can take advantage of by adopting Agile methods:
- Users and stakeholders get to see and experience the system as it unfolds.
- Errors are more easily identifiable improving the efficiency to correct.
- Quality improves because testing starts from the beginning.
- A proper risk process is implemented at the initial stages of development.
Agile QMS for Medical Software Developing Companies
Now that you have learned about AAMI TIR45 and how Agile can play a key role for your SaMD development, let's talk about ways to further enhance production with an effective QMS!
The optimized solution to reduce risk, maintain quality, and accelerate SaMD innovation is by utilizing an FDA-compliant and best-practice conformant medical device quality management application. Sierra Quality Management System (QMS) allows you to have conformity AND compliance in your production process, all while remaining completely Agile.
Sierra QMS is designed for organizations looking to market medical devices in a globally regulated environment. It is built for engineering teams to operate with their preferred tool sets while automating compliance with medical device QMS principles for global markets. Sierra Labs helps both Medical Device and SaMDs developers to build a robust QMS that includes a variety of effective workflow management tools.
Want to breeze through FDA regulations with zero stress?
Check out Sierra QMS today!
It's that simple!

