Learn how to implement Agile into your SaMD development and also stay compliant.
The Agile methodology of software development by continuous iteration is gaining momentum in the regulated product-development market. The Agile methodology in med-tech is a non-linear workflow with multiple process segmentations and iterations. Here, rapid execution gets an upper hand over careful planning and documentation.
At first glance this method might seem to contradict the FDA-mandated, linear, stringent medical device manufacturing norms. But in 2012, the Association for the Advancement of Medical Instrumentation released the AAMI TIR45 guidelines to help developers achieve maximum compliance using Agile methodology, which has in turn helped Agile gain popularity among developers and med-tech companies.
Apprehensions and Possible Solutions
The Agile methodology deviates from the conventional, linear, sequential process-flow approach. However, presenting the final product linearly does not necessitate its developmental cycle to be linear as well.
Technically, it is almost impossible to concretely define one’s requirements without some preliminary, experimental architecture and development work to validate the necessity of a requirement which is well posed and technologically feasible. This highlights the need for optimal iterations for maximum efficiency in process-flow and subsequent product development.
A clear idea is derived upon when cross-functional teams covering all the important domains of software development like planning, designing, developing and validating, work on successive iterations of the product, typically over fixed time periods. Hence, if done in a controlled fashion and executed optimally, the Agile methodology of medical device software designing holds the potential to improve software design and enhance compliance.
The FDA eventually approved of Agile practices in January 2013. Most med-tech software developers are now vouching for Agile in the med-tech industry, with developers and manufacturers endorsing its importance and appropriateness in the business.
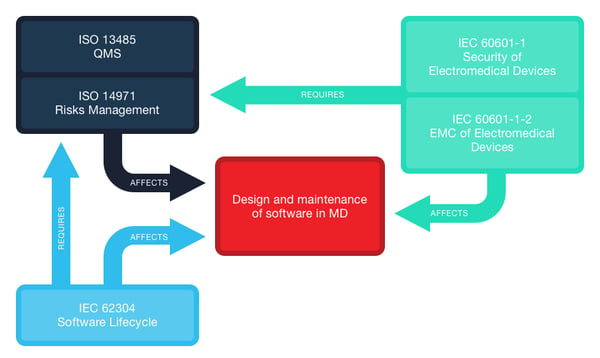
Despite the praise of Agile manifesto for software development, developers are still perplexed. They find themselves in a fix when it comes to judicious and meticulous application of Agile. A decentralized, flexible, iterative model like Agile does not necessarily align with FDA’s stringent regulations on meticulous risk management, mature development processes, and thorough documentation. This makes developers apprehensive about Agile’s compliance with rigid standards like 21 CFR Part 820, ISO 13485, ISO 14971 and IEC 62304.
The AAMI TIR45 attempts to solve these queries and provide clear navigation of adapting to Agile methodology in medical device development.
Key takeaways from the guidance on Agile
The AAMI TIR45 is neither a standard nor a recommended practice, but is instead designed to dig deep into existing standards and find synergies in terms of concepts and practices for the Agile methodology. It provides information in terms of documentation, evolutionary design and architecture, traceability, verification and validation, managing changes and “done” criteria.
The document is titled Guidance on the use of AGILE practices in the development of medical device software. It describes certain pre-requisites for successful implementation of Agile in med-tech firms.
The different prerequisites highlighted in this document are:
|
|
|
|
Incorporate Agile into your Life Cycle Management
There is an overall hierarchical flow of development processes. There are four layers of development according to the Agile framework:
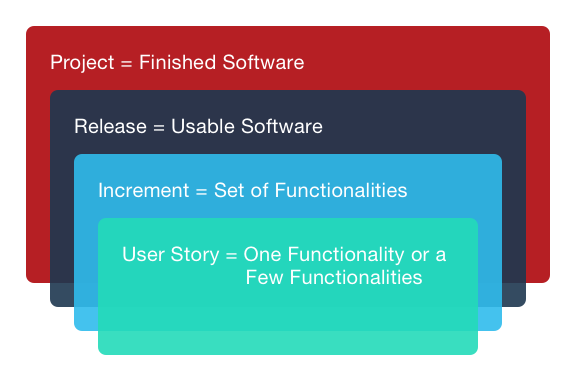 Fig 1: Layers of Development in Agile Methodology
Fig 1: Layers of Development in Agile MethodologyThe activities executed in the layers of functionality are aligned with the IEC 62304 requirements, ensuring that all regulatory requirements are met while employing the Agile methodology in med-tech.
A successful quality management system can be implemented effectively using Agile methods in specific work-environments without compromising on compliance.
Design inputs and outputs are two categories that often confuse developers, as they do not exist in isolation in an Agile environment. This document specifies the design inputs as user stories, along with their defined acceptance criteria. Design outputs are specified as artifacts like architecture and design elements, code, as well as test cases and their results. This document also sheds light on the importance of non-functional requirements for user stories.
Valued Outcomes of Using Agile
The Agile model of med-tech development has its own share of benefits when implemented optimally. Agile helps in developing an initial, experimental architecture and allows for design reviews at the end of each increment, detecting flaws in its earlier stages rather than solely reviewing at the end in a waterfall methodology. This helps reduce cost while also driving a continuous improvement process, enhancing the overall quality of the final product. Continuous incorporation of customer feedback helps in refining the quality parameters even further. Enhancing and prioritizing the backlog throughout the developmental process ensures a closer alignment with customer or market requirements, thus achieving immense precision in an ever-changing market landscape.
And finally, the Agile methodology requires extensive documentation to validate a clear and well-described ‘Definition of Done’ which feeds into the Quality Management System and helps maintain the necessary stringency for successfully tackling compliance audits. To achieve maximum compliance, a lot of med-tech development teams rely on smart tools that provide automated documentation and enhanced collaboration in an accelerated environment that fully supports an Agile environment.

