How to get the most out of Pre-Submission meetings with the FDA.
Now that you have a better overview of a pre-submission, your team is aware of the benefits this tool can have for launching your device(s). It is crucial to also understand what actually happens during the application process before utilizing it. We understand a lot goes into getting an application from submission to approval. We are here to help you and your team be well-informed to gather all the necessary materials before pre-submission.
How to submit an application?
At the time of submitting your application, your team must decide if it makes sense to meet in person, teleconference, or give written feedback. When scheduling an in-person meeting, be sure to provide them with a draft agenda and three available times to give them flexibility for the meeting.
What is the Pre-Submission Review Workflow like?
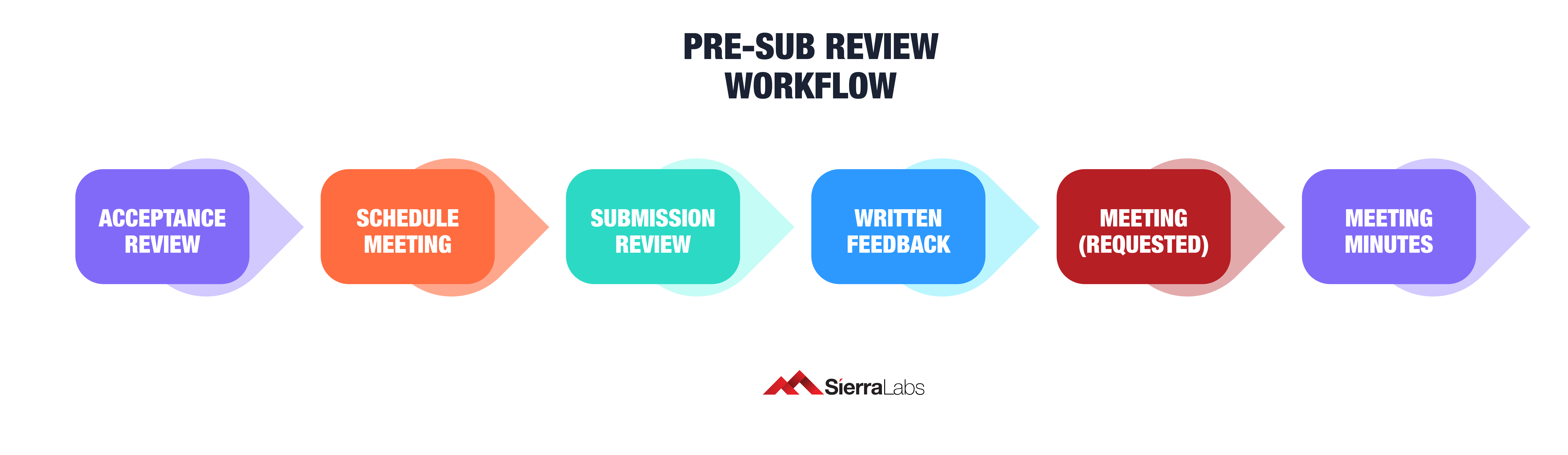
After submitting an application using the FDA’s acceptance checklist, the agency will have 15 days to send you a response, known as the acceptance review stage. If denied for any reason(s), your team is notified with descriptive feedback detailing why their application was rejected.
If the FDA decides to proceed with your application, you should receive written feedback from the review group within 70 days or at least 5 days before when a meeting is requested along with your feedback. Below is the expected stages of a pre-submission process:
What are the potential types of feedback?
The pre-submission meeting is designed to expedite the regulatory process for innovative medical technologies helping shorten total review times and make it easier on all stakeholders involved. The feedback given by the review team of the agency can include questions as specific as device classification. The following is a list of types of feedback according to the FDA:
- Device classification questions
- Intended use:
- Medical condition and population
- Type (qualitative vs quantitative)
- Screening vs Diagnostic
- Point of Care/Home use
- Planned nonclinical studies:
- Precision/Reproducibility
- Stability
- Interference
- Carryover
- Cross-reactivity
- Planned clinical studies:
- Population (adults, pediatric, pregnant, etc.)
- Inclusion/Exclusion criteria
- Sample size
- Clinical sites
- Clinical reproducibility
- Specificity/Sensitivity
- Reference method and/or method comparison
- Statistical analyses
- Software/Cybersecurity/Risk management
- Labeling
What not to do before or during a meeting?
It's important that your team be as specific and clear about your questions before submitting them. Vague or general questions will not yield valuable feedback from the review group. Companies that want to avoid missing any important information during the application process should make sure they ask targeted questions in their application. General questions will only lead to addressing general areas of interest which will result in a longer timeframe for your regulatory journey.
The review team is here to answer all of your regulatory questions but they will not serve as consultants, in other words, they will not discuss specific data with you. Your team should not expect your solution to be right away approved or cleared in any way, however, you can still establish a working partnership with the FDA around your device’s regulatory journey.
What to do after the meeting?
Your team is responsible for drafting minutes or documenting your interactions to ensure a thorough understanding of all regulatory requirements and standards for your device. After the meeting concludes, you have 15 days to submit these minutes back to the agency. The FDA will alert you within 30 days if these draft minutes are final or if they require revisions.
You may disagree with their decisions so there is a timeline your team must follow in order to resolve any disputes. You may ask for amendments or edits of minutes within these 15 days of your meeting. If there aren’t any revisions, we still recommend you review the minutes with your team as the FDA will make these minutes final within 30 days of receiving an email.
There aren't any hard limits on the number of pre-submission meetings you can request for a specific device. If additional questions and topics arise after written feedback is given and minutes are final, then it is beneficial to apply again with your team’s new findings in mind before proceeding into a final regulatory submission.
Simplifying Pre-submission for your team
When submitting a request for medical devices, it is best if you find professionals who can not only help with the process but also plan out your roadmap and assemble all of the correct documentation.
When it comes to navigating the complex world of regulations, there are tools that can make this process easy and stress-free. One such resource is an electronic Quality Management System (QMS), which will help you reach your market goals with minimal worry while maximizing efficiency.
Sierra QMS is designed for organizations that are looking to market SaMDs/ DTx’s/ AIaMD in a globally regulated environment. Its incredible integration capabilities are built for engineering teams to operate with their preferred tool sets while automating the compliance process with SaMD QMS principles for global markets
Want to see how Sierra QMS can help your organization?
Click Here for a Free Consultation!
Ask us anything.

