Understanding the power of utilizing a pre-submission for your medical device.
Is there a way the FDA can give you feedback on your device, before submission? Yes, yes there is! The FDA Pre-Submission is a great service for companies to request feedback from the agency on potential and planned medical devices, biologics, or drug submissions. We found that it tends not to be utilized enough but there are many reasons why you might want this input before submitting your application!
Let’s explore why this tool was created and how you can utilize it for your own medical solutions!
Why was the Pre-submission created?
The Pre-Submission process was created to help companies get ahead of issues before final submission. This can keep your product on track for its development timeline with the foresight of potential problems and discover ways to correct them before the actual submission!
The FDA is always on the lookout for new medical devices to help patients, but they have thousands of applications from companies all over. In order to get their attention and allow you time to study your solution further before making any decisions about whether or not to approve it for use by the public.
What is a Pre-submission?
The pre-submission process allows your team to work with the FDA in an informal setting with the goal of ensuring your team is prepared for questions and concerns around the components used, the development process, and the safety of your product. This ensures everyone working together towards common goals has solid data at hand while minimizing risk factors.
FDA provides feedback to sponsors and applicants in the form of a pre-submission meeting or teleconference. This formal written request for information from FDA is called a "Pre-Sub".
FDA is working with a number of device manufacturers to help them bring their products from concept all the way through production. This process has been streamlined so that it only takes one submission type for most devices, which means less time spent waiting and more focus on getting your product off shelves as soon as possible!
When can you use a Pre-Sub?
The Pre-Sub is a great way to get FDA’s feedback on specific questions in order for you and your team members to know what they need before going forward with product development or submission preparation.
Your team may take advantage of a pre-submission when considering an investigational or marketing application.
- Investigational (IND, IDE, HDE, Master Files, Special Protocol Assessment)
- Marketing (NDA, PMA, BLA, 510k, De Novo)
The information in this document is not applicable to your team when:
- It pertains specifically and solely to identifying general FDA policies or procedures.
- Simple clarification questions that can be answered by staff readily
- Discussion of issues while the submission is under active review
- Appeal meetings
When can you submit?
If your company is considering submitting an application that is classified as investigation or marketing, then pre-submission is the first step. With this submission, you are assured that the FDA will be able to appraise the specifics of the device with ease. The device you are developing could potentially be compliant with the most necessary regulations, but there's always the chance that it won't meet all standards. By providing vital information to the agency, you are giving them a glimpse of the purpose, scope, and timeline of your production process as well as the various steps it's taking to comply with not only a few regulations but up to industry standards.
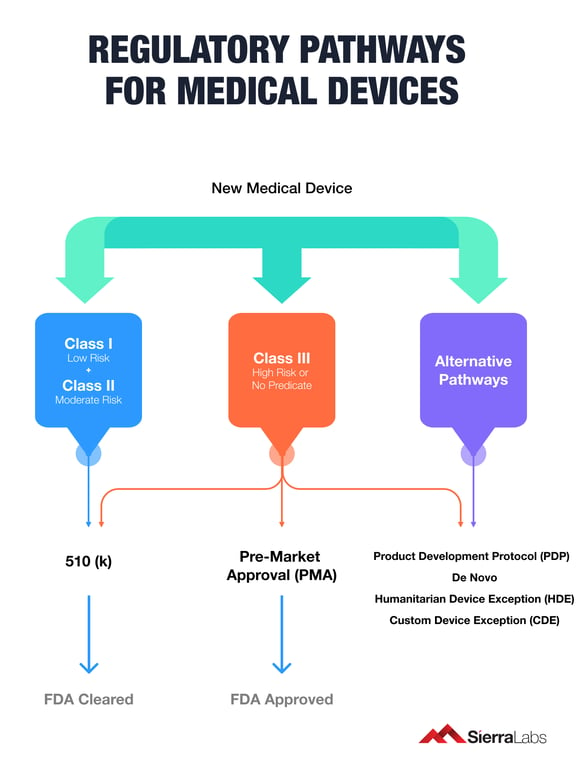
If your device doesn’t fall within the established risk classifications of the agency making it ambiguous which submission pathway to choose, then you should submit a pre-submission to establish one with the FDA.
Click here to uncover which regulatory pathway is right for your medical device!
Even if you are planning a future study or conducting research that will lead to a future application, you should utilize a pre-submission! The FDA can help you navigate the regulatory process by establishing stepping stones for your research.
What to include in a Pre-Sub?
You should submit the following documents and information in your Pre-Submission to the FDA:
- Cover letter that includes the following information:
- Cover Sheet
- Designation of meeting request type
- Manufacturer name & contact information
- Product Name
- Pre-Submission package
- Purpose
- Regulatory History
- Planned Follow-on Submission
- Background Information
- Device Description
- Proposed Predicate Devices (if applicable)
- Intended Use/Indications for Use Statement
- Description of How the Device is Planned to be Used
- Risk Analysis
- Discussion of Relevant Prior Information
- Proposed Study Design
- Specific Questions
- Meeting Attendees
- Proposed Meeting Dates (unless written feedback is requested).
Other requirements include one eCopy and one hard copy of the application. These should be exact clones of each other. Both should be signed electronically and/or by hand.
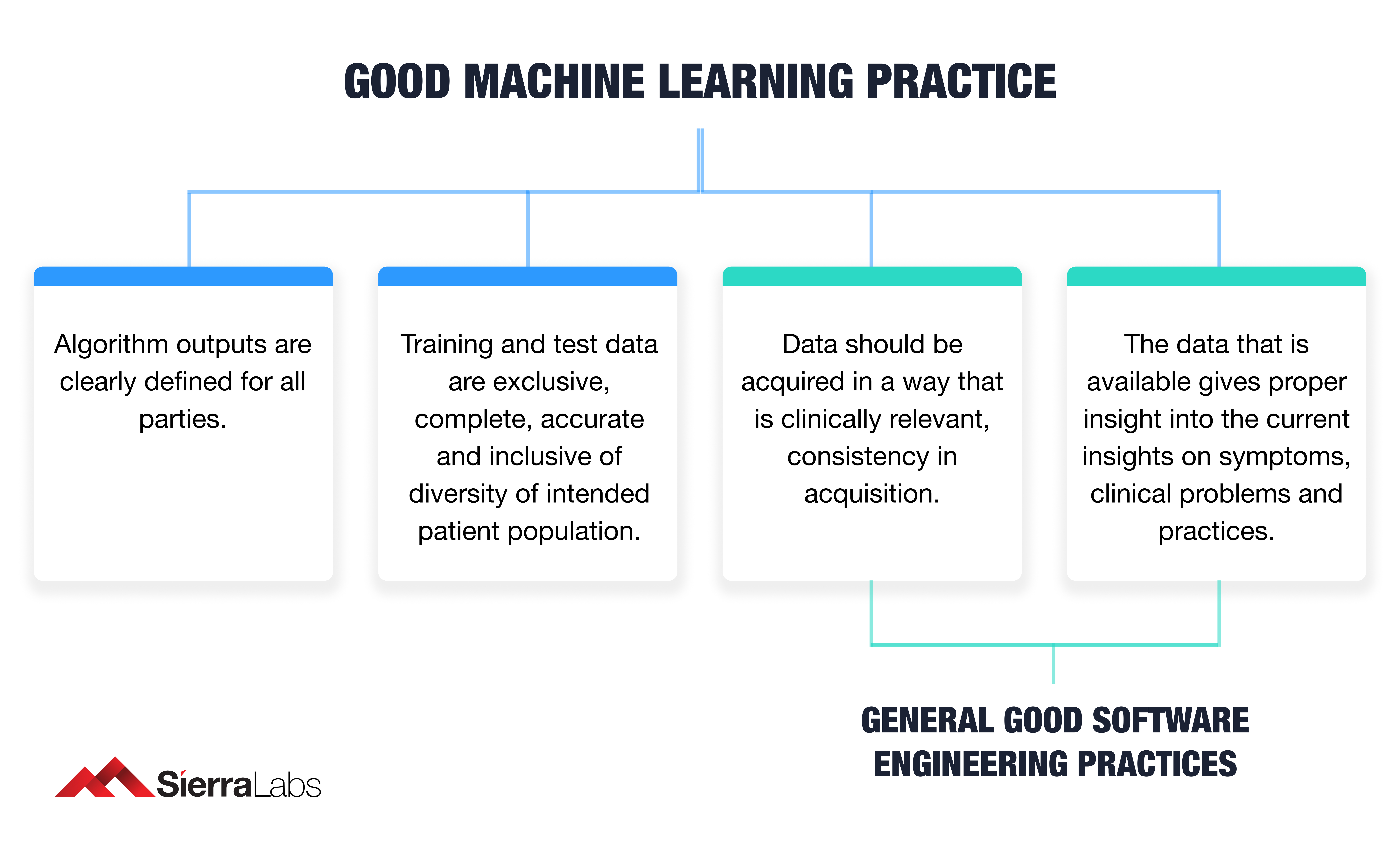
What if I am developing an SaMD and AIaMD?
It is important to understand the expectations of the FDA before developing a SaMD or AIaMD. Especially for software that includes AI algorithms, the pre-submission can provide valuable insights into the agency’s expectations for these types of solutions.
The FDA can specify how your device will be evaluated such as:
- Parameters for product validation with topics for a test protocol
- Performance benchmarks
- Need for clinical validation/acceptance criteria
- Discussions on methods
- Data points for real-world monitoring and analytics.
Simplifying Pre-submission for Your Team
There are a lot of reasons that companies might be hesitant to use the Pre-Submission program, but it's really not as complicated or time-consuming as you may think.
Sierra Labs has experience working with early-stage medical device companies that do not have clear criteria outlined for their own quality processes. We can simplify this process for your team by helping you implement the necessary well-documented procedures for FDA audits and ISO standards.
Here at Sierra Labs, we help companies solidify their regulatory foundation by creating a unique and tailored quality program to meet organizational objectives with complete transparency and visibility across their entire operation.
Does Your Team Need Help With Pre-Submission?
Click Here for a Free Consultation!
Ask us anything.

