Breaking down the basics of Software as a Medical Device (SaMD): risk categorizations and examples.

You may hear the term "SaMD" tossed around in conversation or in literature pertaining to this industry, but do you really understand what it means? In this blog, we will help you dive deeper into what constitutes as an SaMD, go over each of its risk categorizations, and provide examples that will help you understand all of this a little bit better. By the end of this blog, you will not only be comfortable with the term SaMD, but you will also know how to risk categorize your medical device and effectively manage it. Let's get started!
FDA’s guidance on Software as a Medical Device (SaMDs)
International Medical Device Regulators Forum (IMDRF) and U.S. Food and Drug Administration (FDA) release publications to give guidance on Software as a Medical Device and to improve existing regulations.
The notion of SaMDs referred in the guidance published by the FDA in December 2017 was extracted from an IMDRF document in September 2014. IMDRF published this document to offer a framework for regulating different types of SaMD. This publication by the FDA explains that improved regulatory requirements for SaMD are to evolve more.
Software in the Medical Devices Sector
FDA states software in the medical devices sector in three separate entities:
- Software in medical device
- Software used for the maintenance and manufacturing of a medical device
- Software as the medical device
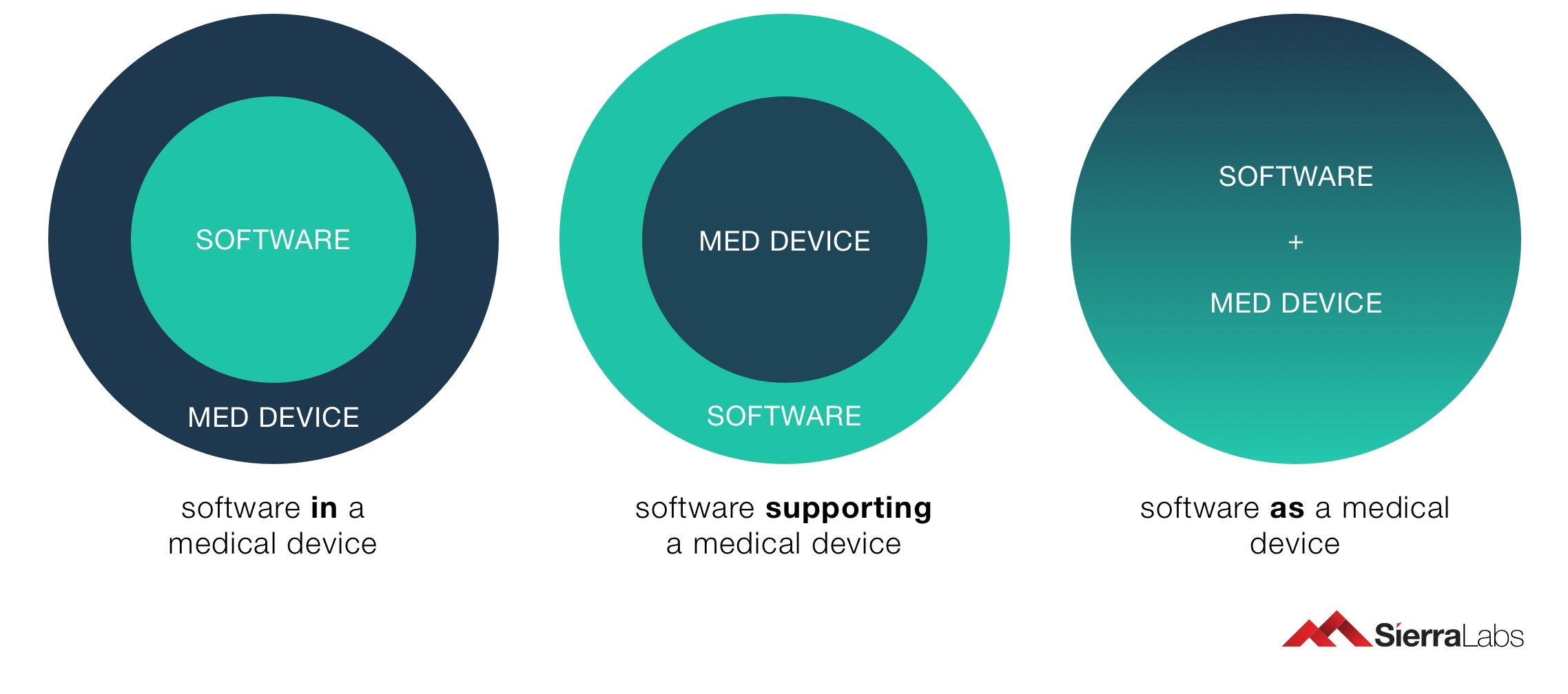
It is not easy to differentiate between SaMD and other software used for medical purposes. The IMDRF and other European directives have therefore further clarified it in definition of medical devices, by adding the term "software" within the definition of medical devices. Any other medical Software may work with other physical devices, but a SaMD must operate on commonly used computing platforms (such as mobile devices) and may be utilized in coordination with different medical devices. If the software is a component of a medical device hardware, it does not fulfill the FDA’s definition of Software as a Medical Device. For further clarification between Medical Device Software vs. Software as a Medical Device, check out Medical Device Software & SaMDs: A Crash Course!
Need a QMS system for your SaMD? Download our Sierra QMS White Paper to learn more!
Framework for Risk Categorization
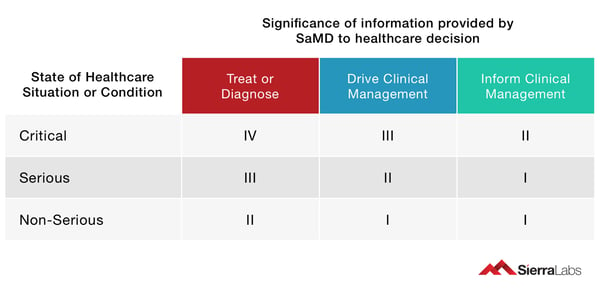
The categories for software medical devices differentiate software applications through two key dimensions:
Significance of information – Devices that are utilized directly in the medical treatment or diagnosis of patient infections are likely to get higher requirements of clinical confirmation, containing both analytical and scientific validity, along with clinical demonstration assessment. Software applications which merely inform clinical management are related with less rigorous validation protocols.
Healthcare situation – Diseases are categorized as non-serious, serious and critical. When a SaMD is utilized as an interference for a serious or critical disease, it must be verified more rigorously compared to one whose envisioned application is in diagnosing non-serious diseases.
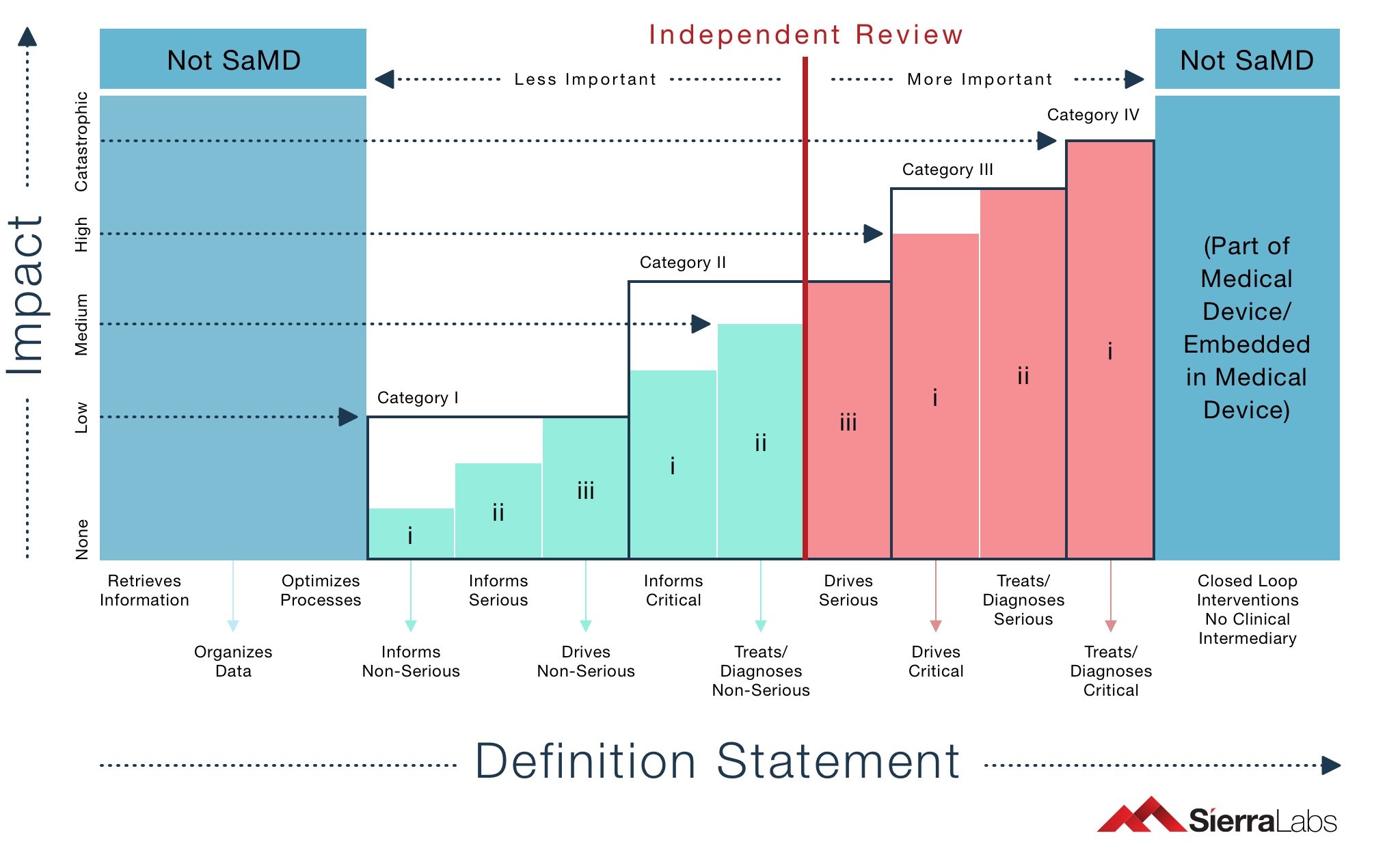
Examples for SaMD categorization
| Impact on Patient: | Health-care Action | Disease Category | Example | Independent Review |
| Category 1 | Inform | Non-Serious | Body mass index and other related calculator based applications in mobile phones or tablets | Optional |
| Inform | Serious | Application to detect Atrial Fibrillation (Abnormal Heart Rhythm detection) | Optional | |
| Drives | Non-Serious | Software that is utilized in clinical aid in the assessment and rehabilitation of joint dysfunction and posture imbalance by active range of motion (ROM) assessment, making use of camera images of joints and postures | Optional | |
| Category 2 | Inform | Critical | Application to identify Myocardial Infarction (Heart Attack) | Optional |
| Treats or Diagnoses | Non-Serious | Application to diagnose a non-serious skin disease with the help of image referencing | Optional | |
| Drives | Serious | Mobile application for the communication between health-care providers and patients while giving birth | Required | |
| Category 3 | Drives | Critical | Software controlling an embedded medical device such as pacemaker | Required |
| Treats or Diagnoses | Serious | Mobile application utilizes high magnification lens and an external UV light source to detect cytologic atypia or cells typical of malignant melanoma | Required | |
| Category 4 | Treats or Diagnoses | Critical | Software which analyze diagnostic image for making treatment decisions (initialization of brain-saving intravenous thrombolytic therapy or interventional revascularization) in patients with acute stroke | Required |
Table 1: Categories of SaMD, corresponding health care actions and examples and the importance of independent review.
QMS For SaMD
Now that you have learned all about risk categorization, let’s talk about ways to actually incorporate risk management into your organization!
The optimized solution to reduce risk, maintain quality, and speed innovation is by utilizing an FDA compliant medical device quality management application for any SaMD company. Sierra Quality Management System (QMS) is a QMS for life sciences that allows you to easily integrate risk management into your production process. The
Sierra QMS is designed for organizations who are looking to market SaMDs in a global regulated environment. It is built for engineering teams to operate with their preferred tool-sets while automating the compliance with SaMD QMS principles for global markets.