How SOC 2 Certification provides security and value for Medical Device Companies.

Over the past few years, data breaches reported to the U.S. Department of Health and Human Services (HHS) have been at an all time high. Healthcare organizations who carry and maintain sensitive patient or caregiver information are particularly vulnerable to these cyber attacks. In this blog, we will focus on how Service Organization Control 2 (SOC 2) is aligned with current best practices for securing data in the healthcare industry and how it can help you meet business objectives.
SOC 2 for Healthcare
In the healthcare industry, nobody wants to work with an organization that can jeopardize their security. The SOCs are a set of compliance standards that were developed by the American Institute of CPAs (AICPA). In a nutshell, SOC 2 avoids putting any business partnerships in jeopardy by ensuring clear security protocols are up to par with standards based on a third-party audit. If you are developing a medical device that in some way handles, maintains, or transfers sensitive data, then SOC 2 can draw up reports that are integral to information security including Personal Health Information (PHI).
What does SOC 2 Provide Your Organization?
Now more than ever, company's developing medical technologies like Software as a Medical Device (SaMD) are committed to demonstrating their quality of security for protected health information (PHI). Securing sensitive data is on the same level of providing quality healthcare services and putting patients’ well being first.
It is crucial to know the criteria that a third-party inspector might be looking for in your organization when awarding a SOC 2 certification. SOC 2 audit is dependent upon the principles of the Trust Services Criteria (TSC) which is comprised of 5 distinct points that outlines the standards necessary to keeping sensitive data private and secure while it’s in transit or at rest.
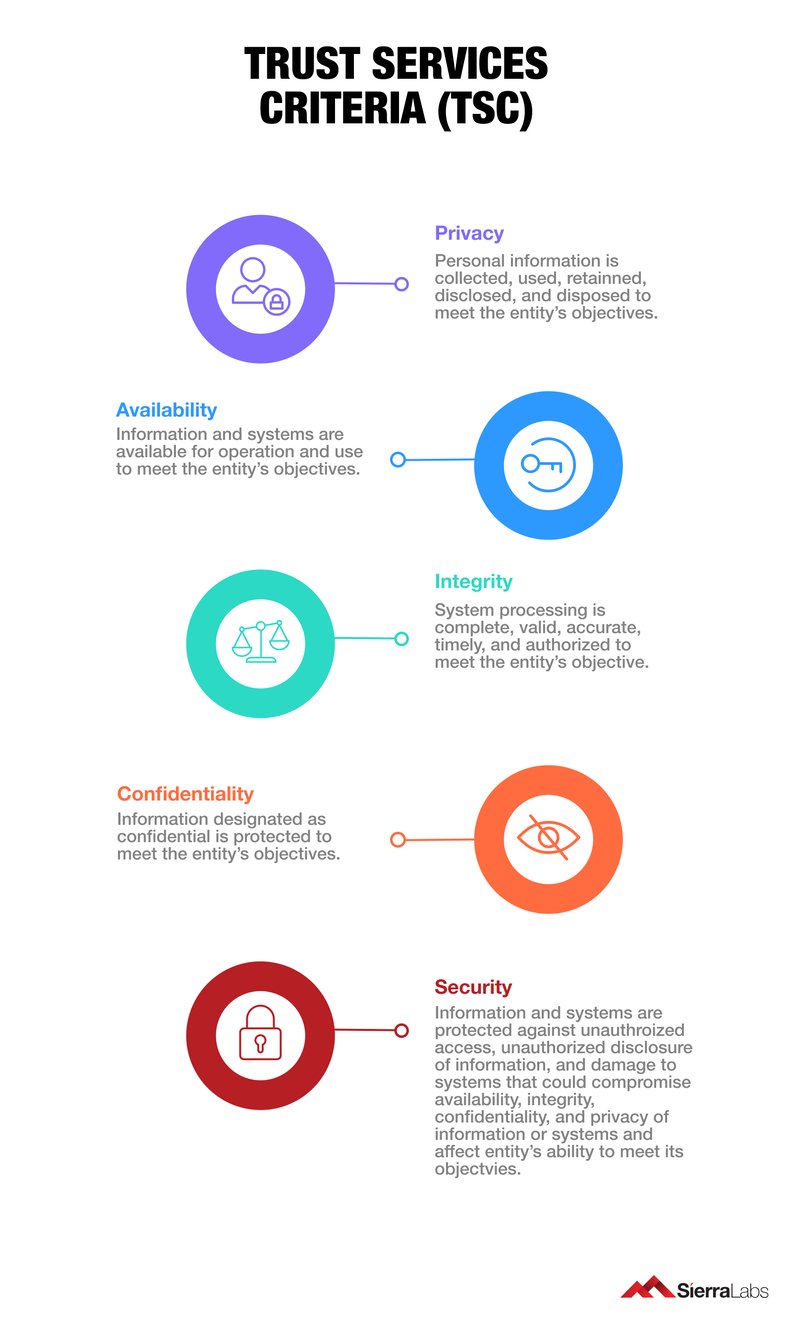
Competitive Advantage for your Organization
Undergoing a SOC 2 audit demonstrates that your company can provide sufficient security and translate well into achieving business goals. SOC 2 Certification will boost your company’s reputation and provide a competitive advantage when seeking a partnership with a healthcare organization. Think of it as a branding tool! You can market your organization as having the highest level of security for your systems increasing reliability in the healthcare organizations you partner with.
Get your Organization Audit-ready
To qualify for SOC 2 compliance in this area, an organization must demonstrate that they protect and handle personal information securely. SOC 2 compliance is about showing organizations that you’re competent and are taking steps to ensure your customers’ data is secure. Here is what we can offer your business and team.
With Sierra Labs, you can fully understand the specific requirements and options to develop audit-ready actionable policies and procedures to become SOC 2 Certified. Our team will create a regulatory roadmap for your specific business size and type to uncover critical points that need to be improved for audits or assessments.
Sierra Services is the first step to achieve regulatory compliance, speak with our regulatory experts and avoid the hassle of compliance obstacles for your medical device's journey to market. We will guide you every step of the way to ensure your team feels safe and confident for inspections and submissions.
Want to see how you can achieve SOC 2 Certification?
Click Here for a Free Consultation!
It's that simple.

