How Emergency Use Authorization is shaping the regulatory landscape of Healthcare during the COVID-19 pandemic.

In light of the Coronavirus pandemic, the availability of medical products has never been more vital than now. The recent shortages of medical supplies have caused disruption for medical facilities, hospitals, and the healthcare system as a whole.
Yet, there are various entities both public and private working to supply the necessary resources to treat this pandemic. Key players in this space are:
- FDA
- Manufacturers
- Pharmacies
How is FDA speeding up approval time?
Reviews usually take months to complete. Due to coronavirus’ rapid spread, the FDA has expedited its review process from months to a week.
In 2017, the FDA finalized a guideline known as the Emergency Use Authorization of Medical Products and Related Authorities (EUA). The EUA outlines how products would be reviewed under a public health emergency.
Medical products include drugs, devices, and biological products. With less strict revisions on medical products, doctors and caretakers are able to diagnose, treat, or even prevent life-threatening conditions during this crisis.
Hand Sanitizer Production Amid COVID-19
The absence of various medical products has affected every key player in this space. One of the first products to deplete was Hand Sanitizer. Pharmacy retailers like Walgreens, Rite Aid, CVS, have felt the shock of this pandemic.
The FDA enacted the EUA for Hand Sanitizer due to its unprecedented depletion. This EUA has lifted production approval for various manufactures outside of the medical space in hopes of producing more sanitizing gel. Breweries and distilleries can now provide support during this pandemic in the form of manufacturing hand sanitizer, also known as ethyl alcohol.
Are you a Brewery or Distillery looking to make hand sanitizer to help out the cause?
If so, we can help! Tell us about it.
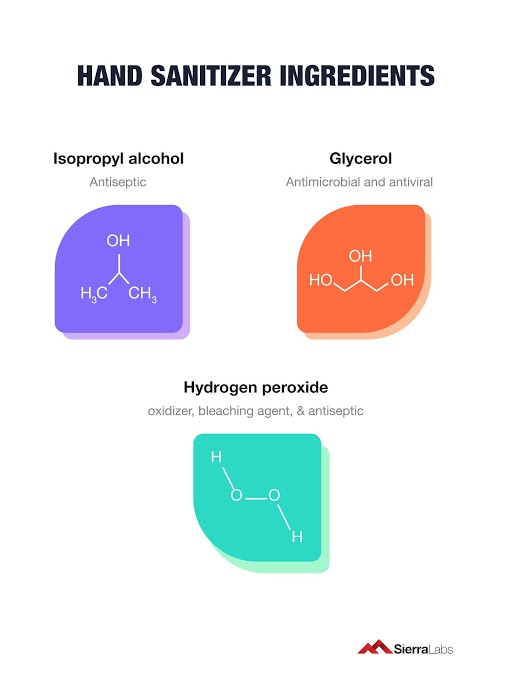
Despite these laid back federal regulations, manufacturers must follow the World Health Organization’s (WHO) recipe for hand sanitizer without shifting away from the main components. (alcohol, glycerol, hydrogen peroxide, and water). The FDA has also provided images of what information must be displayed on the bottles for them to make it to the retailer shelves.
Despite these laid back federal regulations, manufacturers must follow the World Health Organization’s (WHO) recipe for hand sanitizer without shifting away from the main components. (alcohol, glycerol, hydrogen peroxide, and water). The FDA has also provided images of what information must be displayed on the bottles for them to make it to the retailer shelves.
Lighter Regulations for Compounding Pharmacies
Prior to EUA, the FDA required compounding pharmacies to have a prescription for a specific patient in order to make a new compound. The FDA has lifted these regulations and are allowing pharmacies to modify medications for specific patients as they see fit.
An example of a modification is taking a drug that is only sold as a pill, and converting it into a liquid so that it can be swallowed more easily, or taken at a dosage that is not commercially available.
Where is the liability?
While the EUA has been a strong regulatory force in reducing supply shortages during this public health crisis, liability remains a concern.
Under EUA-authorization, do manufacturers and health care providers have any protection from tort liability if anything goes wrong with these products?
There is the Public Readiness and Emergency Preparedness (PREP) Act, which provides immunity from tort liability for these manufacturers and health care providers primarily because the EUA still provides a minimal set of regulations for companies to follow. However, the scope of protection of this PREP Act isn’t unlimited and also hasn’t been tested in any court.
How can we help?
As the COVID-19 pandemic remains ongoing, the FDA will continue to roll out new policies, guidelines, and recommendations to ensure there remains the proper quantity of medical products while also ensuring their quality.
Here at Sierra Labs, we want to do our part in helping stop the spread of coronavirus. We do this by providing support to any and every company that wants to bring a COVID-19 solution to the market.
Whether you are a medical device company looking to design coronavirus testing kits, an SaMD looking to develop a software to help COVID-19 patients, or a lab conducting clinical trials that needs to connect with patients during this time, we can help!
As mentioned earlier in this blog, we can also help companies that are not typically in this industry (such as Breweries, Dispensaries, etc.) that are looking to make high-demand products such as hand sanitizers, masks, etc.
If you or your business have any ideas or products to help fight COVID-19, we want to support you in getting your solution to the market in the quickest and easiest way possible. If you need any help navigating regulations or understanding the regulations that apply to you in deploying your product to market during this crisis, we’d love to help you in any way possible.
Unfortunately, time is of the essence for getting your COVID-19 solution to the market faster to help save human lives.
Let's fight COVID-19 together. Please feel free to reach out with any questions!
Working on a COVID-19 Solution?
We can help expedite your product to market!
Ask us anything.

