A simple breakdown of the Software Pre-Certification Program and how it affects your company.

If the idea of a Software Pre-Certification is confusing to you, think of it in relation to getting TSA Pre-check. Yes, the process of enrolling in TSA Pre-check is lengthy, tiresome, and tedious. But after going through all of the regulatory steps and getting approved, your overall experience at the airport becomes a lot smoother and more efficient, while still maintaining overall airport safety and security. This analogy helps to understand the basis of FDA’s Software Pre-Cert Program; you take on the extra steps/work to get pre-certified so that your future development process can become much more time and cost effective.
Let’s break everything down as simply as possible to help you understand:
- What exactly is Software Pre-Cert
- Its goals and advantages
- Whether or not your company should be involved in the testing of this program (pros and cons)
Timeline of FDA’s Software Pre-Cert Program
Just this year, the FDA announced its official launching of the Software Pre-Cert Pilot Program, but planning and ideation of this program began back in 2017. After a year or two of refining, version 1.0 of the Working Model for the Software Precertification Program (Pre-Cert), along with a corresponding Regulatory Framework and a Test Plan, was released and is currently being tested in the field. The results of this years’ tests will determine whether this will go to congress to be passed and integrated into the Software as a Medical Device (SaMD) production process, thus replacing the lengthy submission process that is currently in place.
Timeline:
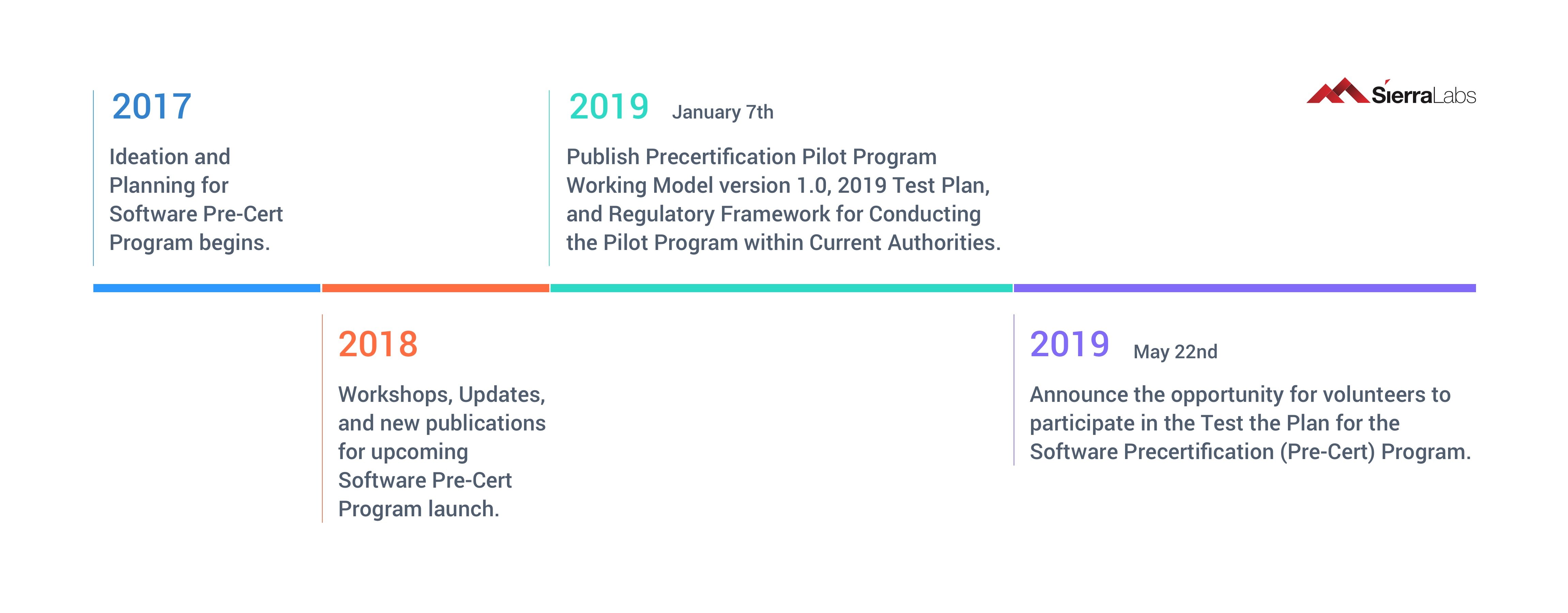
Fig. 1: Timeline of FDA Pre-Cert Updates
What is Software Pre-Cert?
The Software Pre-Cert Program is an attempt by the FDA to expedite the time-to-market process for Software as a Medical Device (SaMD) while maintaining product quality and patient safety. Currently, it takes about 90 to 180 days to complete the FDA medical device approval process, and depending on delays or application edits, this timeframe can increase or even double.
There needed to be a certification process that could keep up with the innovation speed of softwares. Softwares are forever evolving, upgrading, and going through multiple iterations, thus there should be a certification process that reflects this.
The Software Pre-Cert Program aims to do this by certifying a company and its software products based on their 5 “excellence principles”:
- Patient safety
- Product quality
- Clinical responsibility
- Cybersecurity responsibility
- Proactive culture
Once a company is certified, it can go through multiple product iterations without having to undergo the entire certification process again, and can instead partake in “streamlined reviews” (or in some cases, skip the review all together) as long as the product is determined to be low-risk.
The Software Pre-Cert Program has 4 main components:
- Excellence Appraisal: Adherence to the 5 excellence principles
- Review Determination: Risk classification
- Streamlined Review: Accelerated review process
- Real-World Performance: Post-market product evaluation (further detailed in Fig. 2 below)
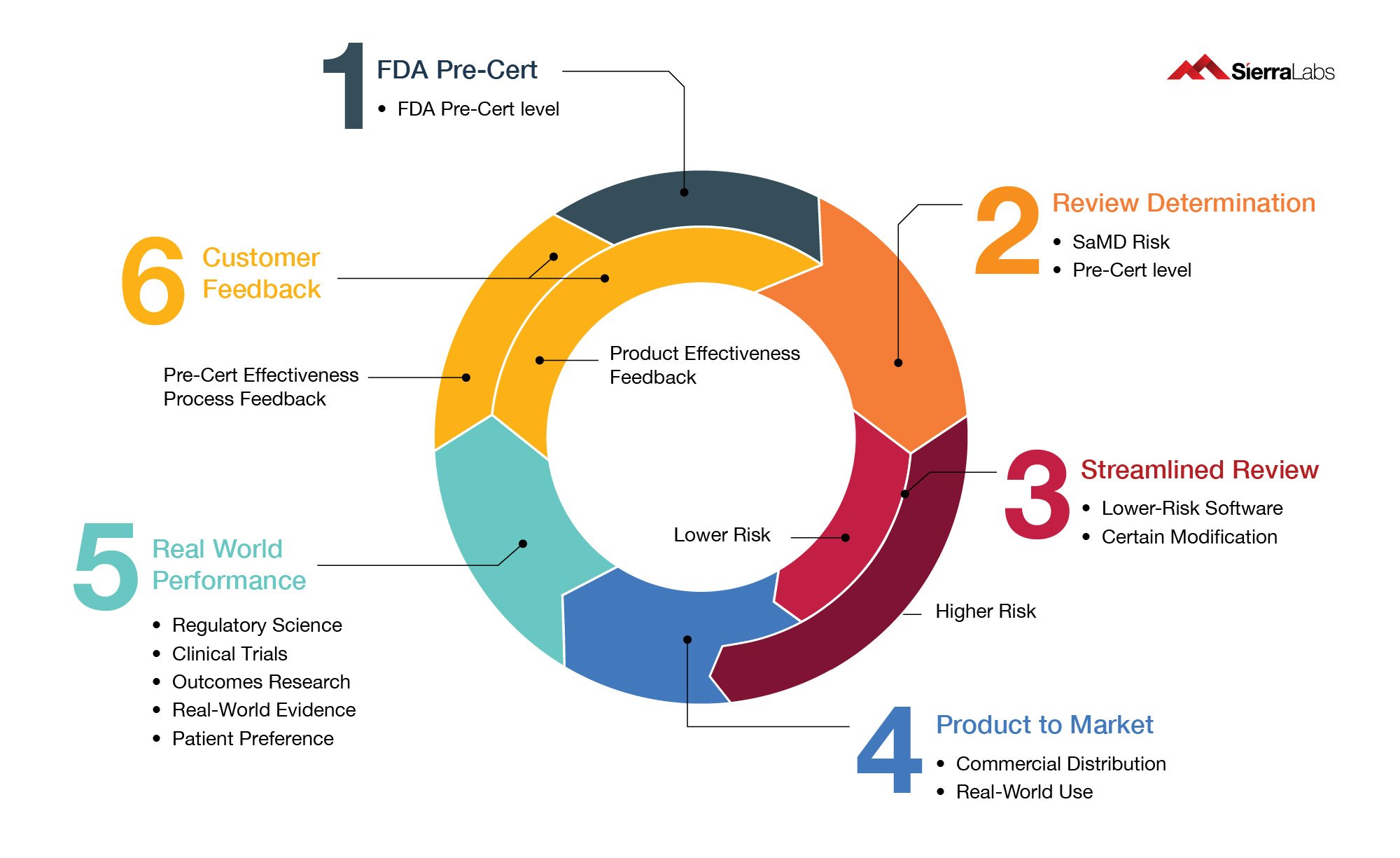
Fig.2: FDA Pre-Cert Concept
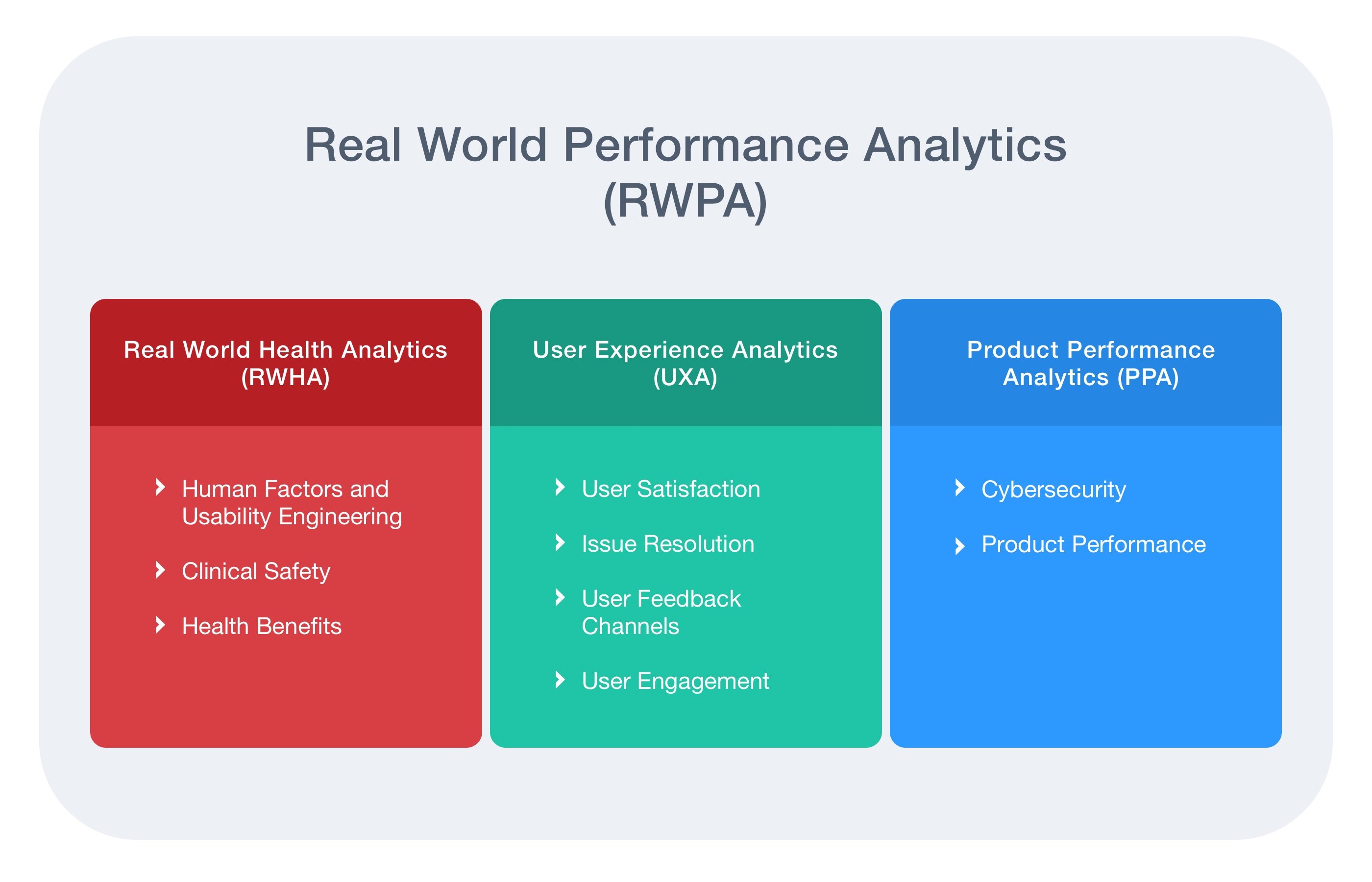
Fig. 3: Real World Performance Analytics (RWPA)
Goals and Advantages of Pre-Cert
The goals of the Software Pre-Cert Program according to the FDA are to:
- Benefit companies with a “precertified” status and provide the opportunity to utilize streamlined premarket review and leverage real-world postmarket data.
- Leverage and use information and data from all available sources, thus increasing efficiency without compromising safety.
- Enable a modern and tailored approach that allows for multiple software iterations in a shorter timeframe.
- Ensure high-quality software products through the entire product lifecycle, due to the enabling of companies to showcase their organizational standards, quality, and excellence.
- Adapt key elements and measure based on the effectiveness of this program.
The advantages of the Software Pre-Cert Program consist of the following:
- Efficiency
- Safety
- Lower manual overhead (for both the FDA and the participating company)
- Fewer times having to complete the certification process (only pre-cert once, unless there are large scale changes)
- Fewer failed submissions and more frequent FDA feedback (earlier course correcting and decreased development costs/time)
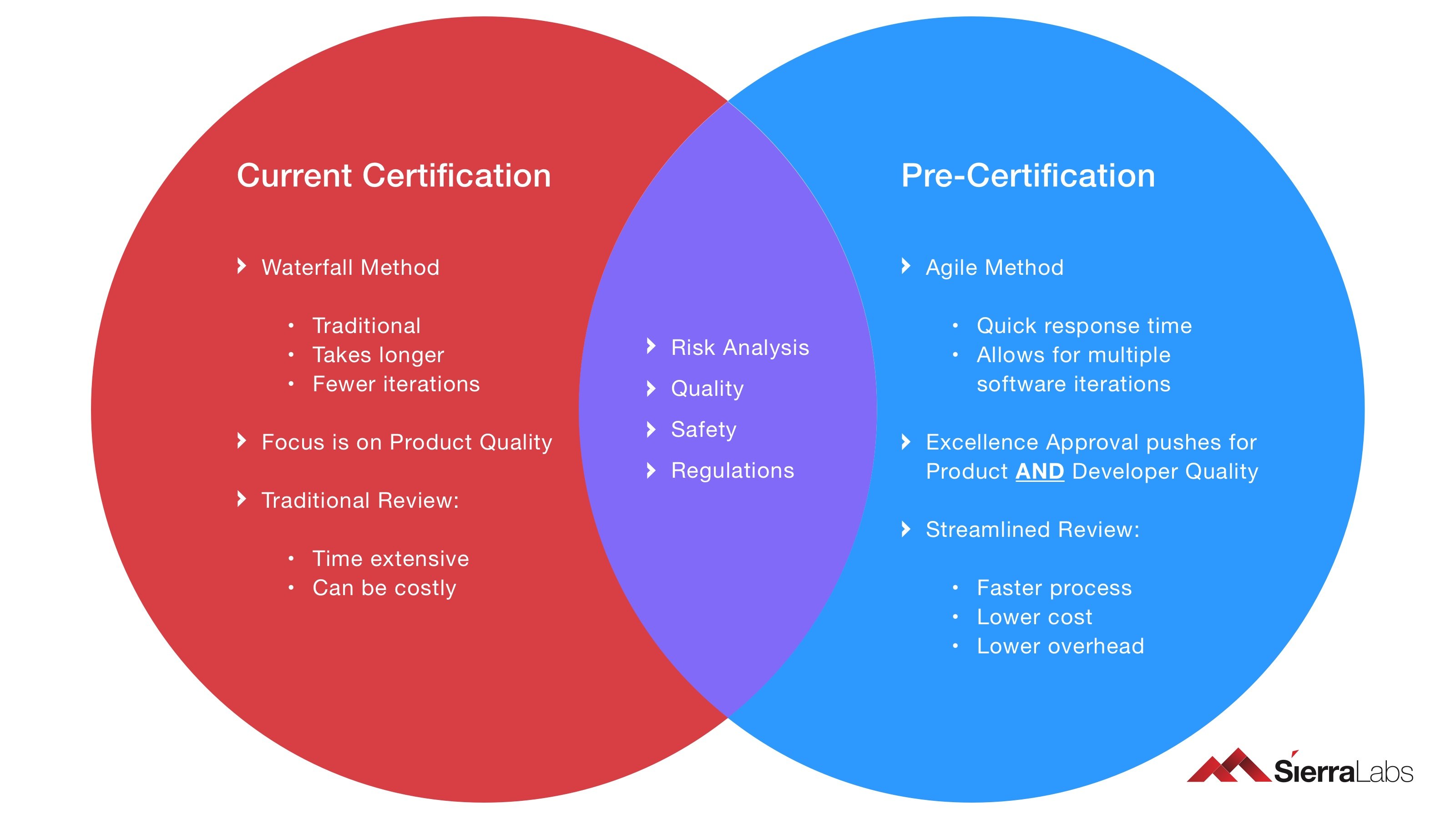
Fig. 4: Current Certification vs. Pre-Certification Venn diagram
Should your company participate in the 2019 Test Plan for the Software Pre-cert Program?
Well, it depends on the size of your company and how many resources you have to spare. In order to ensure patient safety, all companies that are participating in the Pre-Cert Pilot Program will be required to complete the normal 510(k) or De Novo submissions, in addition to undergoing the Pre-Certification process. This will eat up a lot of your time, effort, and resources.
Pros of participating:
- Have a voice in the formation and foundation of this critical industry change.
- Stay competitive and up-to-date with the latest industry developments.
- Can help your business stand out as an industry leader.
Cons of participating:
- Timely
- Costly
- Pre-Cert might be less useful to companies producing very few products/releases (sticking with the analogy at the beginning of the blog, this would be like going through the lengthy process of applying for TSA Pre-check to only fly once a year).
To sum it up, participating in the Pre-Cert Pilot Program may be advantageous for midsize-enterprise companies that can spare the time and resources, but may be costly and not worth the time/cost for smaller companies and start-ups.
Quality Management System for Medical Software Developing Companies
Now that you have learned all about the FDA Pre-Cert Program for medical device software applications, let’s talk about ways to breeze through both traditional and Pre-cert requirements with an effective QMS!
The optimized solution to reduce risk, maintain quality, and accelerate innovation is by utilizing an FDA compliant and best practice conformant medical device quality management application. Sierra Quality Management System (QMS) allows you to have conformity AND compliance in your production process.
Sierra QMS is designed for organizations who are looking to market medical devices in a global regulated environment. It is built for engineering teams to operate with their preferred tool-sets while automating the compliance with medical device QMS principles for global markets. Sierra Labs helps both medical device software developers and SaMDs developers to build a robust QMS that includes a variety of effective workflow management tools.
Want to see how Sierra QMS can help your organization breeze through both traditional & Pre-cert submissions?
Download our free White Paper to learn more!

